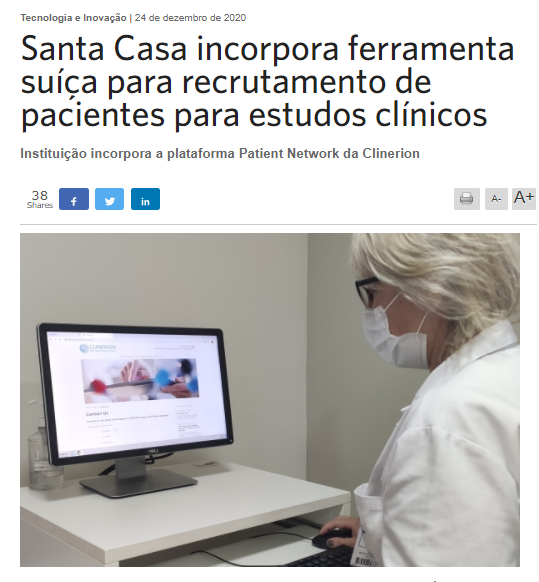
Press article: Santa Casa incorporates Swiss tool for recruiting patients for clinical studies (Setor Saúde, translation)
The following article is translated from the Portuguese and was originally published in Setor Saúde.
Go to the original article >
Institution incorporates Clinerion's Patient Network platform
The Santa Casa de Porto Alegre currently conducts 110 clinical studies sponsored by the pharmaceutical industry in the areas of cardiology, oncology, nephrology, pneumology, infectious diseases, pulmonary hypertension, pediatrics, endocrinology and intensive care. These are studies that seek new treatments, able to control patients' symptoms, cure and prevent disease, and restore people's health. In order to facilitate the capture of patients eligible to participate of these studies, Santa Casa incorporated the Patient Network platform, which combines sponsors' study protocols with anonymous patient data in the hospitals of Clinerion, a Swiss-based company that accelerates clinical research and medical access to treatments for patients.
At Santa Casa, the tool is integrated with Tasy, a medical record system that gathers information from all patients in the nine hospitals that make up the institution, in order to facilitate the participation of these patients in clinical studies.
“Clinical research is aimed at improving the quality of life of people. Participation in the research reaches a greater number of people who may benefit, either through treatments aimed at their recovery, as well as improving health problems,” says Antônio Kalil , medical director of Santa Casa.
As explained by the Teaching and Research coordinator at Santa Casa, Roberta de Almeida da Silva, before the implantation of tool, which is being conducted by iHealth - a company focused on serving the health informatics sector - the search for patients for participation in clinical studies was manual, through electronic medical records and the ICD: “The results, many times, were compromised due to possible record failures, in addition to taking much longer, when in fact the process should be finished with agility.”
International patient privacy rules and data security
According to Santa Casa, the process involves the analysis of Big Data provided by Clinerion, leveraging real-time data from electronic health records that meet international regulations on patient privacy and data security, enabling the Teaching and Research sector of Santa Casa de Porto Alegre the necessary support to constant expansion in their research.






























































































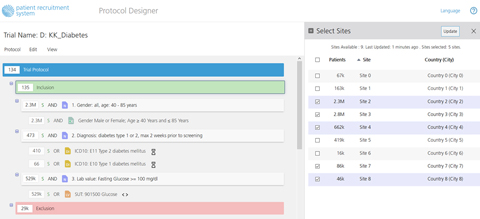 A new look for the Protocol Designer.
A new look for the Protocol Designer.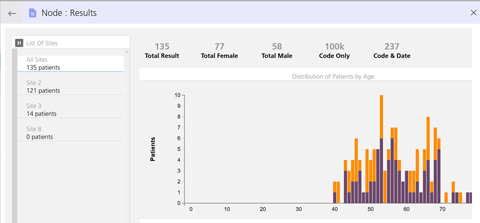 Results in graphical format by age and gender.
Results in graphical format by age and gender.





















