For real-world evidence (RWE), Clinerion’s Patient Network Explorer offers access to real-world data (RWD) queried and aggregated directly from the de-identified, aggregated electronic health records (EHRs) of millions of patients around the world via Clinerion’s network of partner hospitals. It applies intelligent, patented informatics methodologies to extract and interpret information from large amounts of electronic health records and from six dimensions of EHR data: demographics, diagnoses, treatment, medication and laboratory test result values, as well as time. Access to this data allows detailed data analysis of patient journeys and outcomes for health economics and outcomes research (HEOR).
Clinerion RWD services:
|
RWD RADAR: an incidence timeline per EHR field code >
|
Real World Analytics: analytical services on derived data >
|
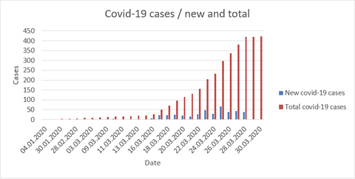 |
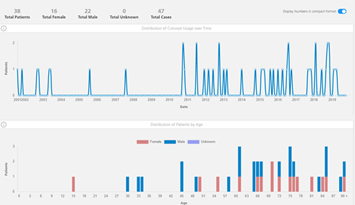
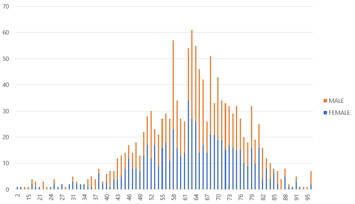
This report is a snapshot of statistics derived from Patient Network Explorer data*, and contains information on:
|
Real-world evidence can illuminate patient journeys and outcomes. While it can never replace Randomized Clinical Trials (RCTs) for evaluating efficacy (and risks) of new treatments, RWE does also reflect real-world treatment situations better than the controlled environments of RCTs. The goals are the achievement of better quality evaluations, representative data, and external validity, while balancing costs and resource utilization.
Clinerion’s Patient Network Explorer harnesses real-time patient data for research, to validate real-world evidence claims and to provide valuable information to support patient care, while respecting patient privacy. It allows users to generate RWD for evaluating and comparing treatments, identifying efficiencies, assessing risks, and implementing proven solutions.
The platform interrogates retrospective data and generates new prospective data for RWE studies, surveillance, eCOA, sentinel and cross-sectional studies, feasibility studies, screening for risk management or health-care system evaluation, among other traditional or innovative study designs.
- Epidemiologists can count on reliable data to understand diseases, unmet needs, treatments and outcomes
- Health authorities can better assess and manage risks and realize benefits
- Payers and HTA bodies have evidence they can trust and can make better decisions
- Clinical researchers and drug developers can expedite R&D and control development costs
- Researchers can access rare or difficult-to-access patient populations
- Pharma and researchers can secure external validity for their cohorts
- As a result, patients can access better health care options.
Clinerion’s state-of-the-art technical platform connects major hospitals around the world, allowing access to the data of millions of patients in real-time. This powerful platform also queries data from a variety of external databases, allowing a focused search of patient characteristics or treatment patterns, as well as with patient, physician and site outreach:
- Query of EHR patient data including demographics, diagnoses, medication, laboratory values and procedures undertaken, longitudinally.
- A global network of partner hospitals in more than 21 countries (and counting!).
- Data in real-time & de-identified/unlinked from identifiers.