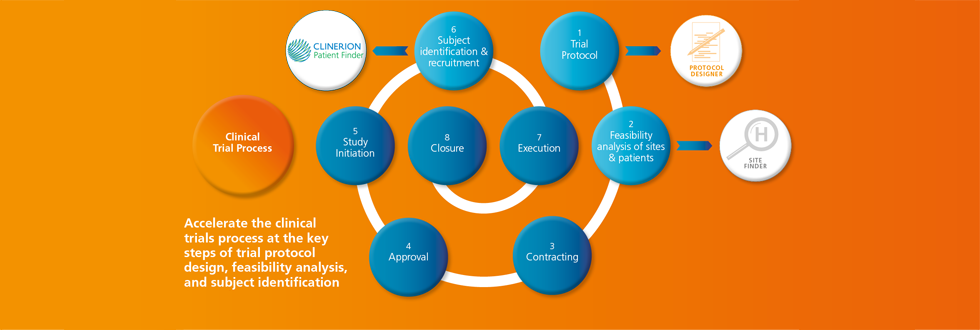
Towards the end of 2014, one of the largest biotech companies in the world started using Clinerion's technology solution for an ongoing dyslipidemia clinical trial.
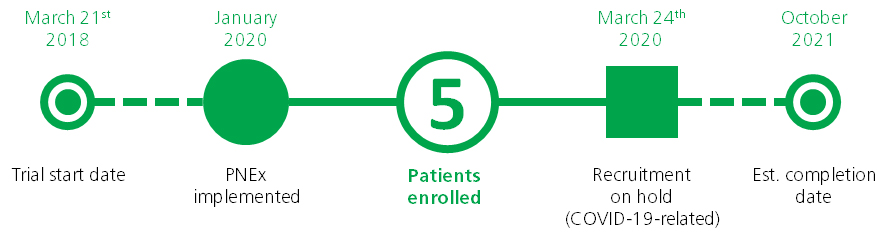
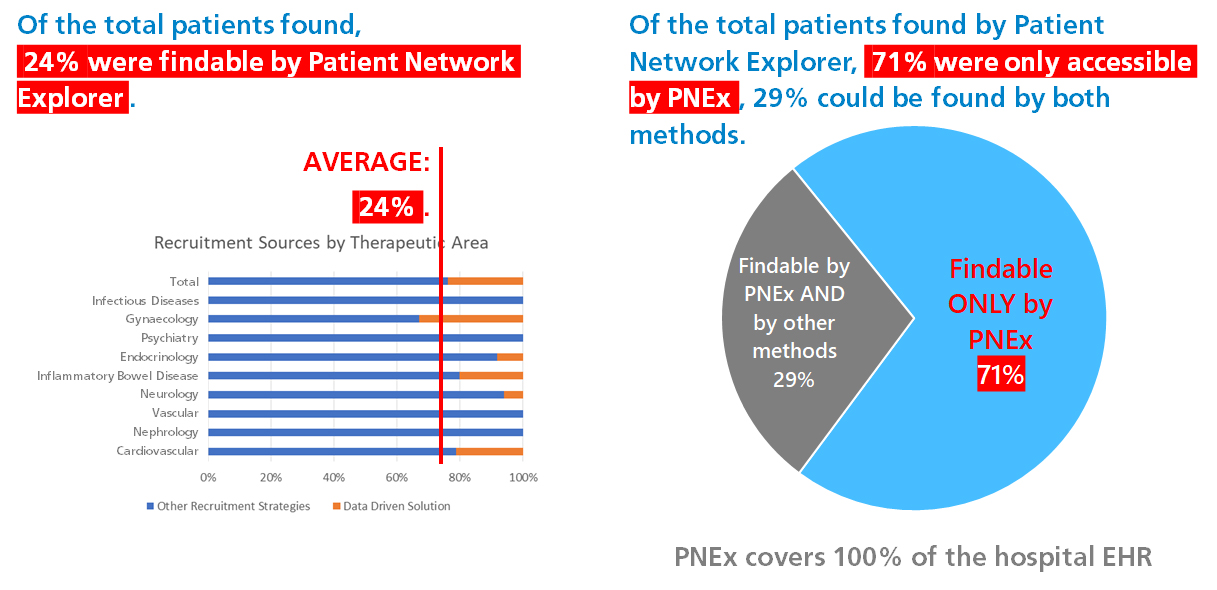
Three months into the trial the study team had only enrolled about a third of the required number of patients. After activating Clinerion, the PI was able to reach the remaining 64% of her commitment within only a month and a half! This result was achieved by only validating 15% of the patient candidates identified by Clinerion. The recruiting process had an early closure due to this high performance of the clinical trial site.
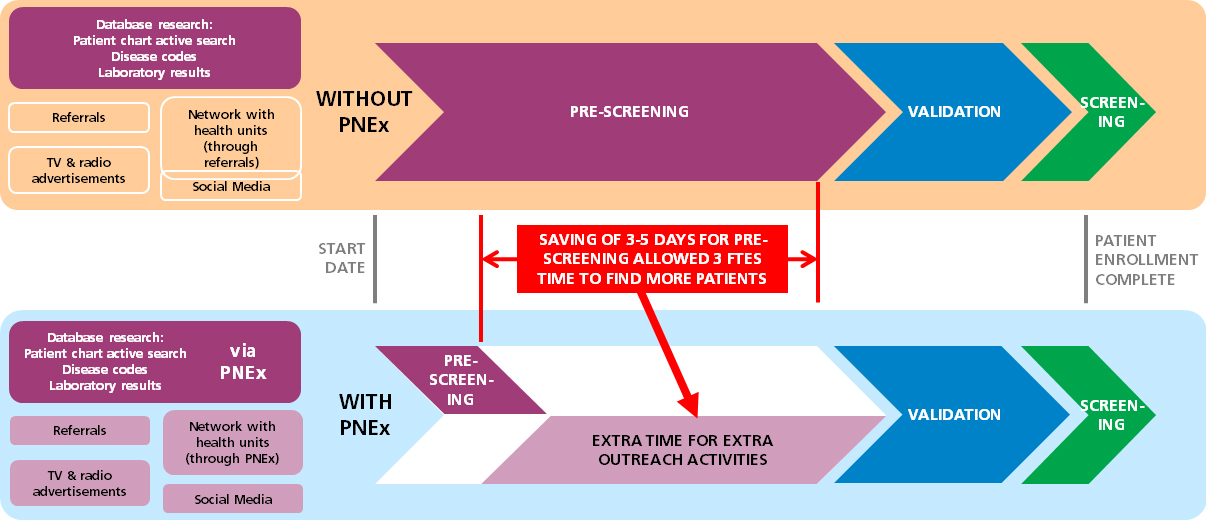
One of the study team members reported: “Normally it takes hours to screen the archives for one eligible patient. However, with such an online tool we spent much less time finding patients and consequently had more time for other tasks. This had a great positive impact on our study quality!”
Published as: ”Case Study: Patient Search and Identification for Biotech Study”, Clinerion, October 9, 2015.